238 new MDSAP document inclusions coming soon:
Our new MDSAP document inclusions will bring the total amount of MDSAP related documents to 265 within platform. Document numbers in each country will be as follows:
- Australia: 111 documents
- Japan: 75 documents
- Canada: 62 documents
- Brazil: 11 documents
- WHO: 6 documents
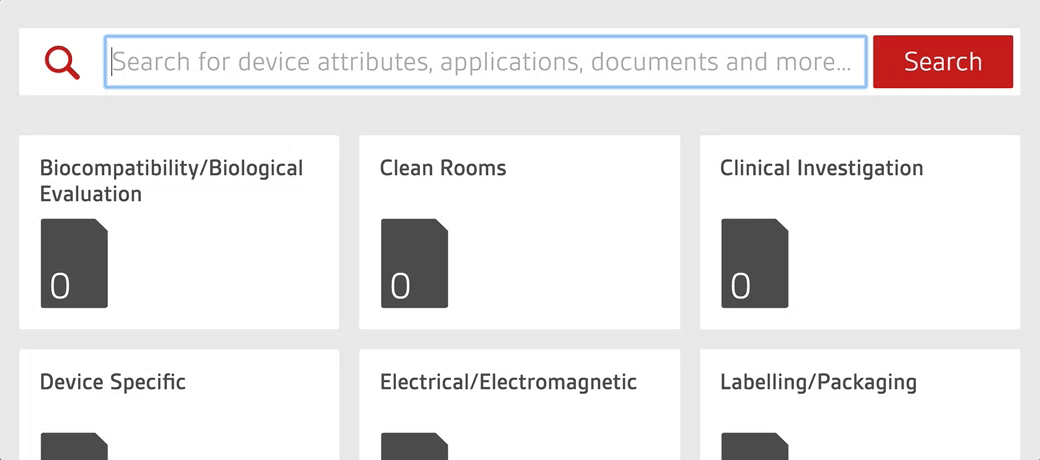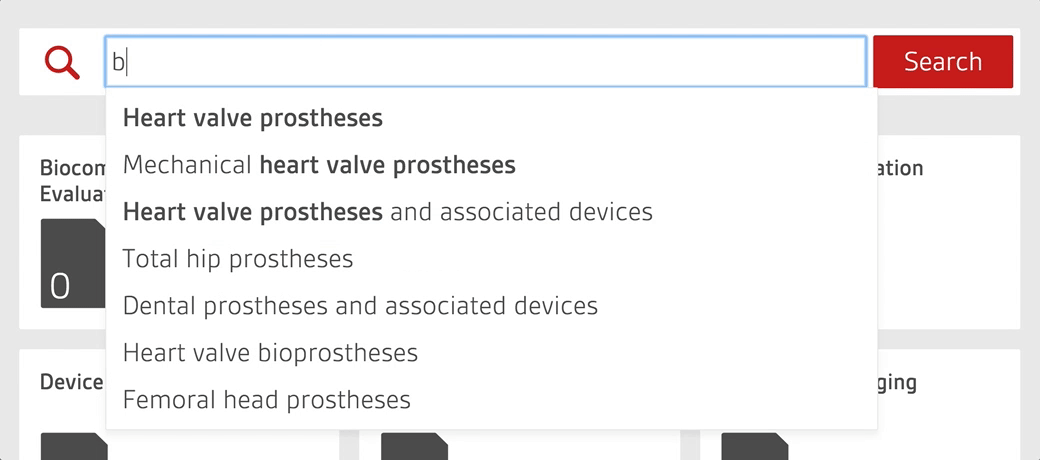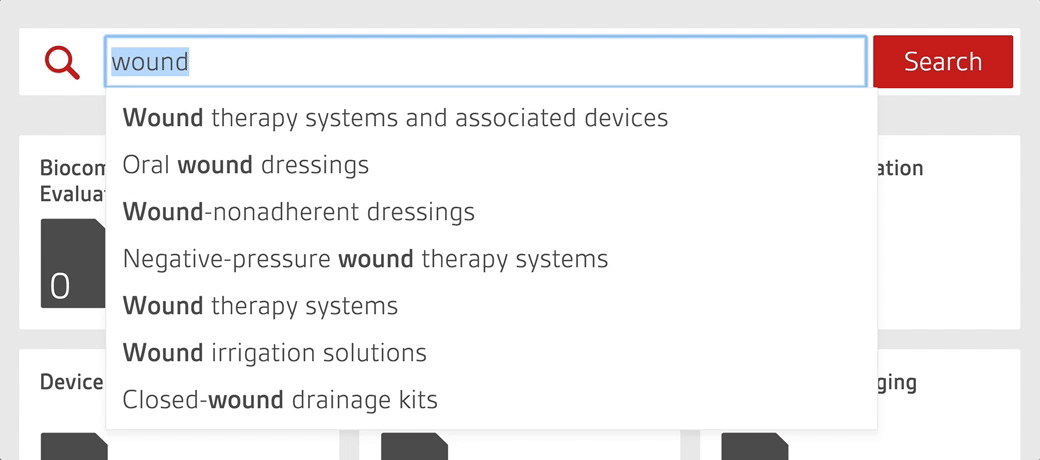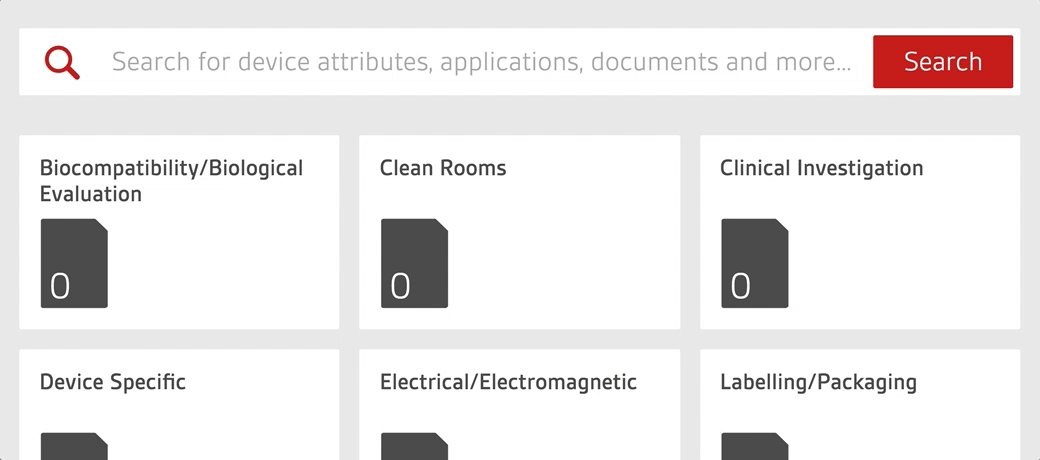
Access over 7500 medical device standards and regulations with Compliance Navigator
- 7500+ medical device standards and regulations
- 1400 + FDA recognized standards
- Advanced warning of standards and regulatory changes
- Gap analysis and commentary written by BSI experts
- Quickly find and access relevant documents through GMDN search functionality
BSI Compliance Navigator helps medical device manufacturers achieve state of the art through high quality guidance and simplified compliance management, all on one platform. Save time, maximise resource and reduce risk. Now offering limited time free access so you can experience all of the Compliance Navigator features at no cost. Gain access to thousands of essential standards and regulations, including BSI, ASTM, CLSI, AAMI and more.
Need more information? View our regulatory and standards content in more depth by viewing our brochure