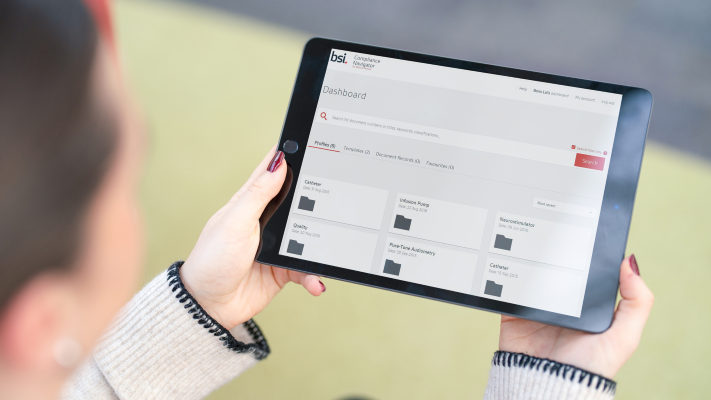
Access over 7500 medical device standards and regulations with Compliance Navigator
BSI Compliance Navigator is an all one regulatory platform that helps medical device manufacturers manage UK, EU and MDSAP requirements. The online tool helps manufacturers discover, organize and respond quickly to changes in the regulatory landscape and mitigate these risks.
- Reduce the risk of recall with real-time and advanced notifications direct to your inbox about document changes.
- Save time with built in gap assessments and expert commentary and on changes to documents relevant to you.
- Search by device or keyword to find every relevant standard for your product portfolio in seconds.
- A single licence provides simultaneous, unlimited user access, globally.
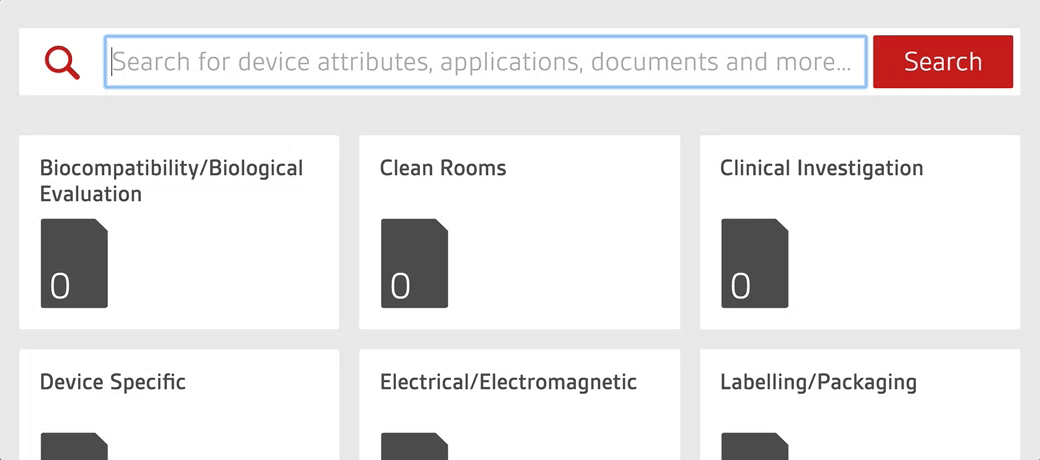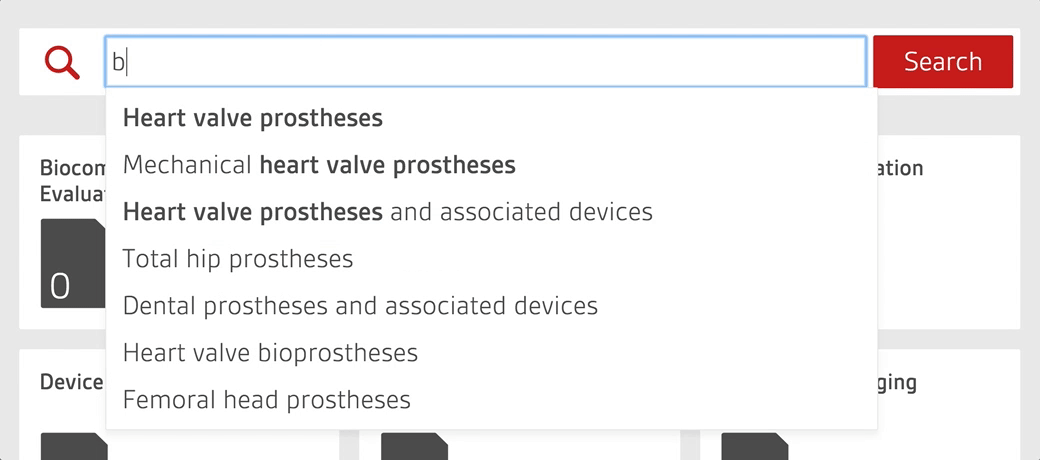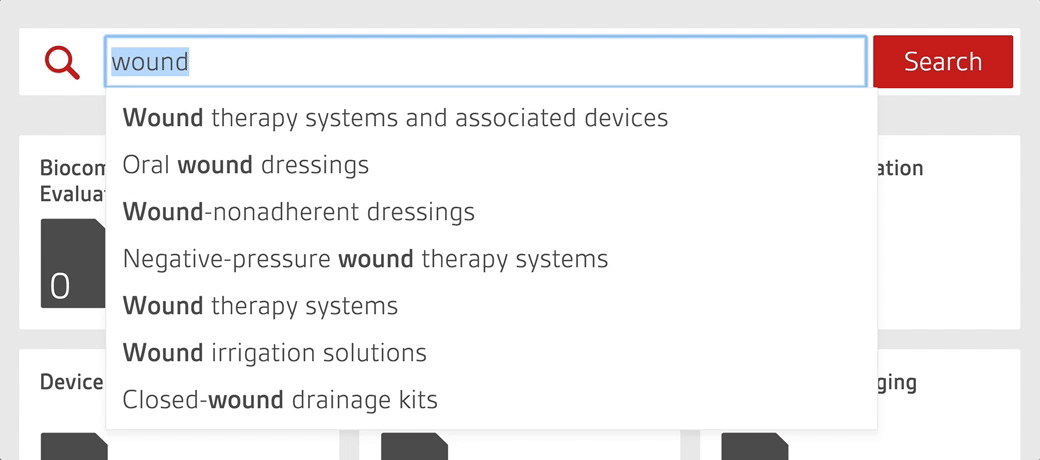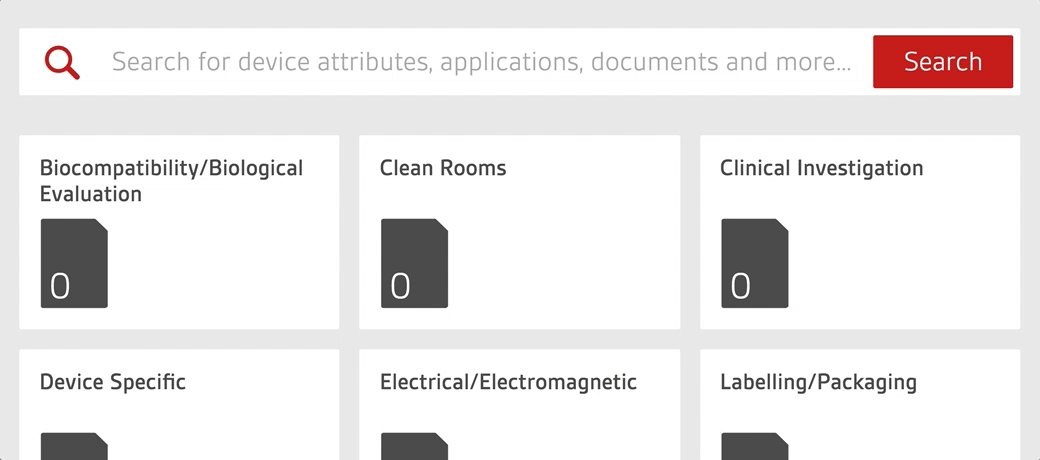
Work smarter with the only platform designed by regulatory experts to help manage your compliance process
BSI’s Compliance Navigator is the smart, simple way to manage your regulatory information for Medical Device and In Vitro Diagnostic products with UK, EU and MDSAP requirements. Stay ahead of regulatory updates, reduce adoption gaps, and accelerate market access through easy interpretation and implementation of high level standards and regulations.
It couldn't be easier to get started
We fully understand the difficulty in remaining compliant in what is an ever-evolving regulatory landscape. BSI are committed to mitigating the regulatory challenges faced by medical device manufacturers. Our team will provide a platform demonstration and offer a free trial, so you and your team can use Compliance Navigator free of charge before making a decision on a subscription with us.