Work smarter with the only platform designed by regulatory experts to manage your compliance process
BSI’s Compliance Navigator is the smart, simple way to manage your regulatory information for Medical Device and In Vitro Diagnostic products with UK, EU and MDSAP requirements. Stay ahead of regulatory updates, reduce adoption gaps, and accelerate market access through easy interpretation and implementation of high level standards and regulations.
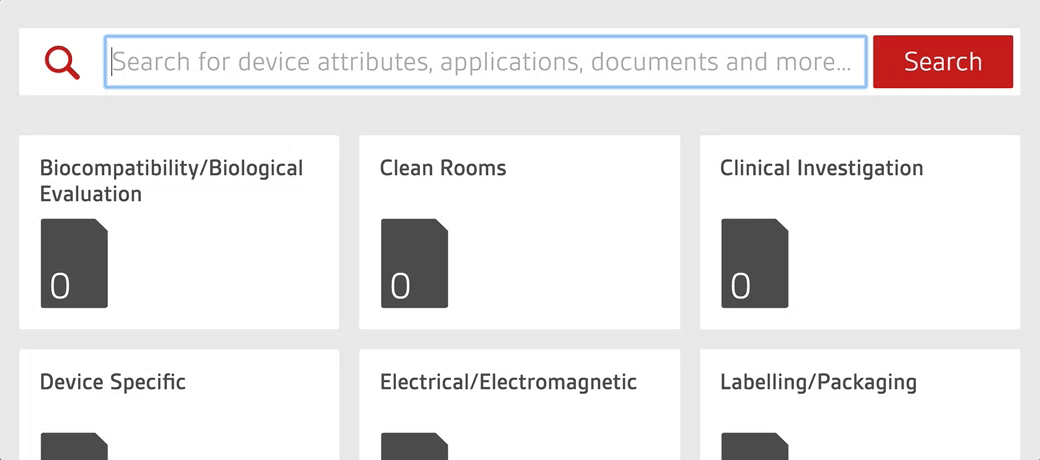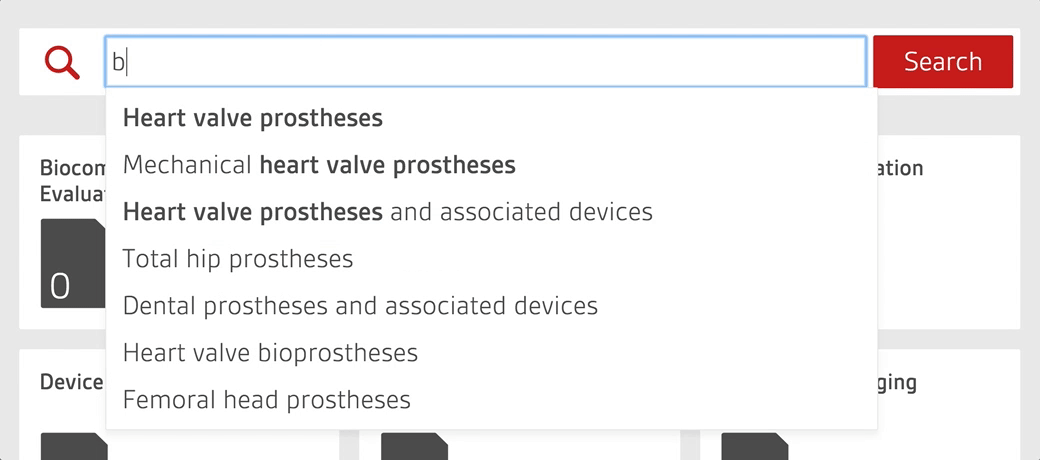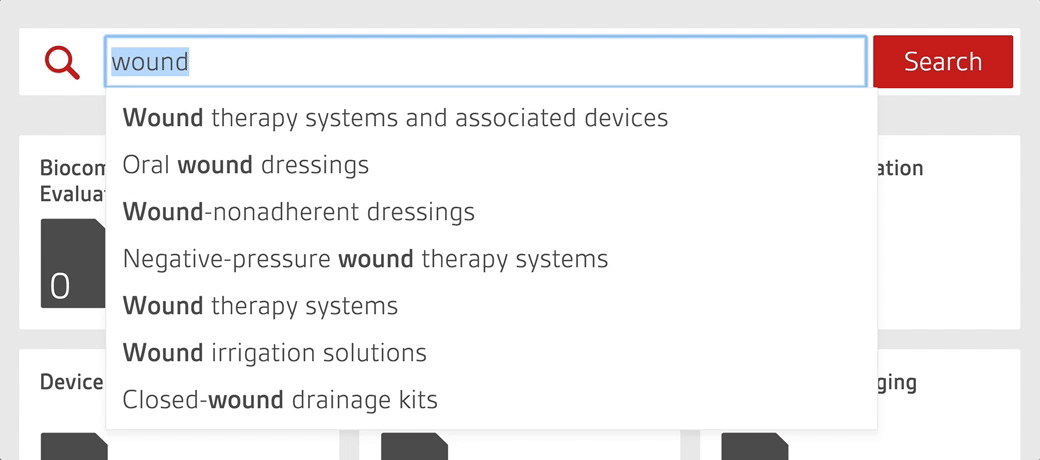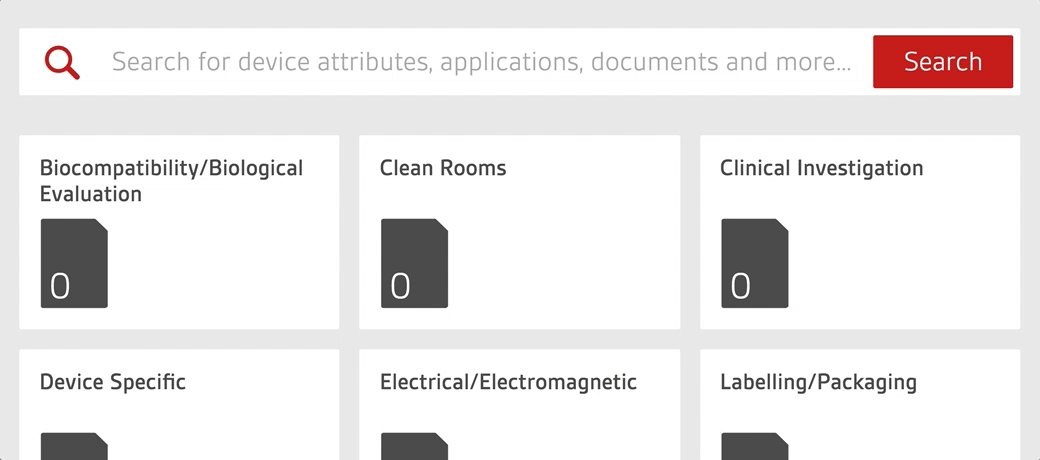
Access over 7000 medical device standards and regulations with Compliance Navigator
- 7000+ medical device standards and regulations
- Advanced warning of standards and regulatory changes
- Gap analysis and commentary written by BSI experts
- Quickly find and access relevant documents through GMDN search functionality
- Unlimited user access with each license for seamless collaboration across teams
It's time to say goodbye to stressful compliance
We fully understand the difficulty in remaining compliant in what is an ever-evolving regulatory landscape. BSI are committed to mitigating the regulatory challenges faced by medical device manufacturers. BSI Compliance Navigator holds over 7,000 documents essential for medical device compliance, in multiple jurisdictions across the world. From tackling the MDR transition, to applying for 510(k) clearance or PMA, Compliance Navigator is your partner in managing medical devices regulation and standards management. With unlimited user access available, there's no better time to try. Request a free trial today via the form to see how we can help!